- Article
- Source: Campus Sanofi
- 23 Oct 2023
Cardiovascular outcomes for diabetes patients Praluent® (alirocumab)


In patients with a prior CV event, Praluent® demonstrated a higher absolute risk reduction in MACE in patients with diabetes than in those with prediabetes or normoglycaemia1*
*Data from a prespecified analysis
~90% of patients in Odyssey Outcomes were on high-intensity statins and 29% of them had diabetes at baseline (99.2% of these had type 2 diabetes run in)2.
In the placebo group, the risk of MACE at median follow-up 2.8 years was approximately twice the risk in patients with diabetes than in patients with normoglycaemia (HR 2.09, 95% CI 1.78–2.46, p<0.0001) or prediabetes (HR 1·90 95% CI 1·65–2·17, p<0.0001)1.
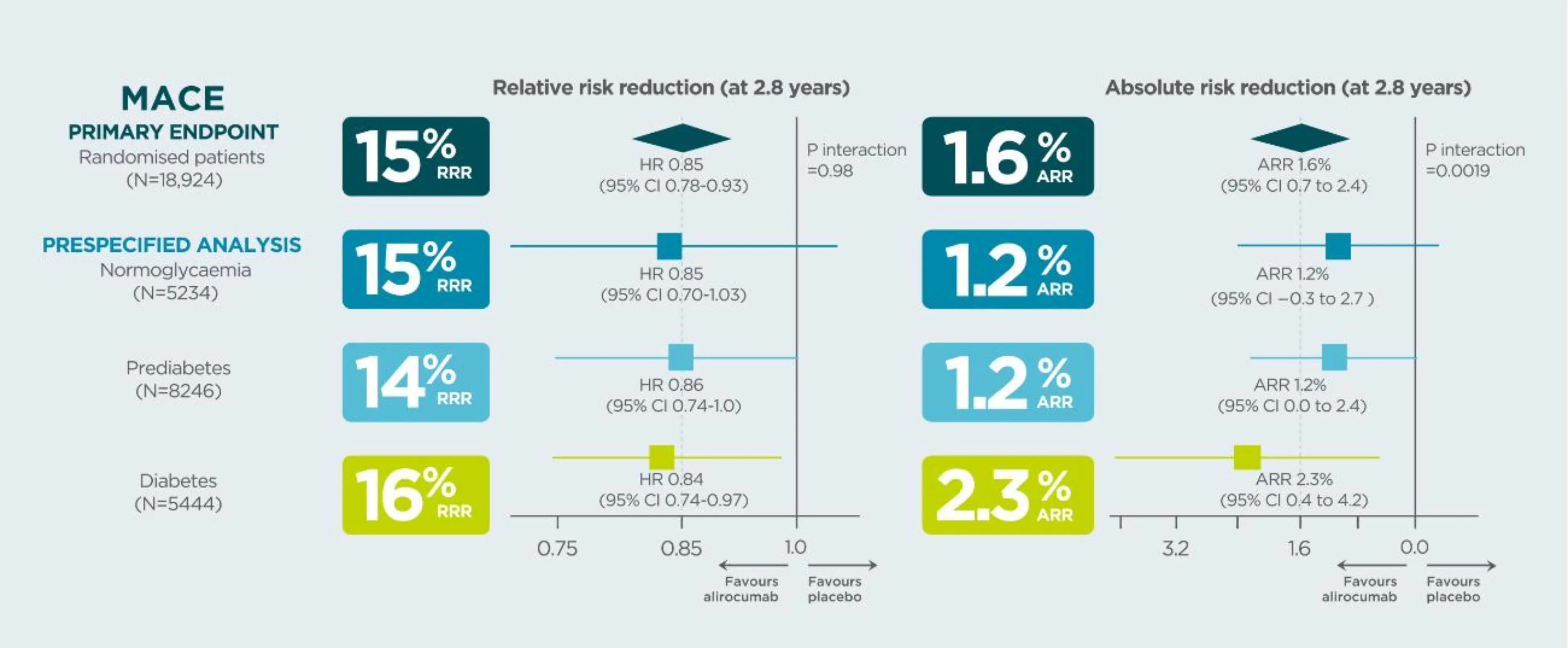
Study design: ODYSSEY OUTCOMES was a randomized, double-blind, placebo-controlled phase 3 study. Patients with a recent MI or unstable angina, and on high-intensity statin (40 or 80 mg atorvastatin or 20 or 40 mg rosuvastatin, or maximally tolerated dose of one of these agents) +/- other lipid-lowering therapy but not at predefined target LDL-C were enrolled. Patients were classified by glycaemic status at baseline (normoglycemia, prediabetes, or diabetes) defined on the basis of patient history, review of medical records, or baseline HbA1c or fasting serum glucose, and risk of new-onset diabetes among those without diabetes at baseline1,2.
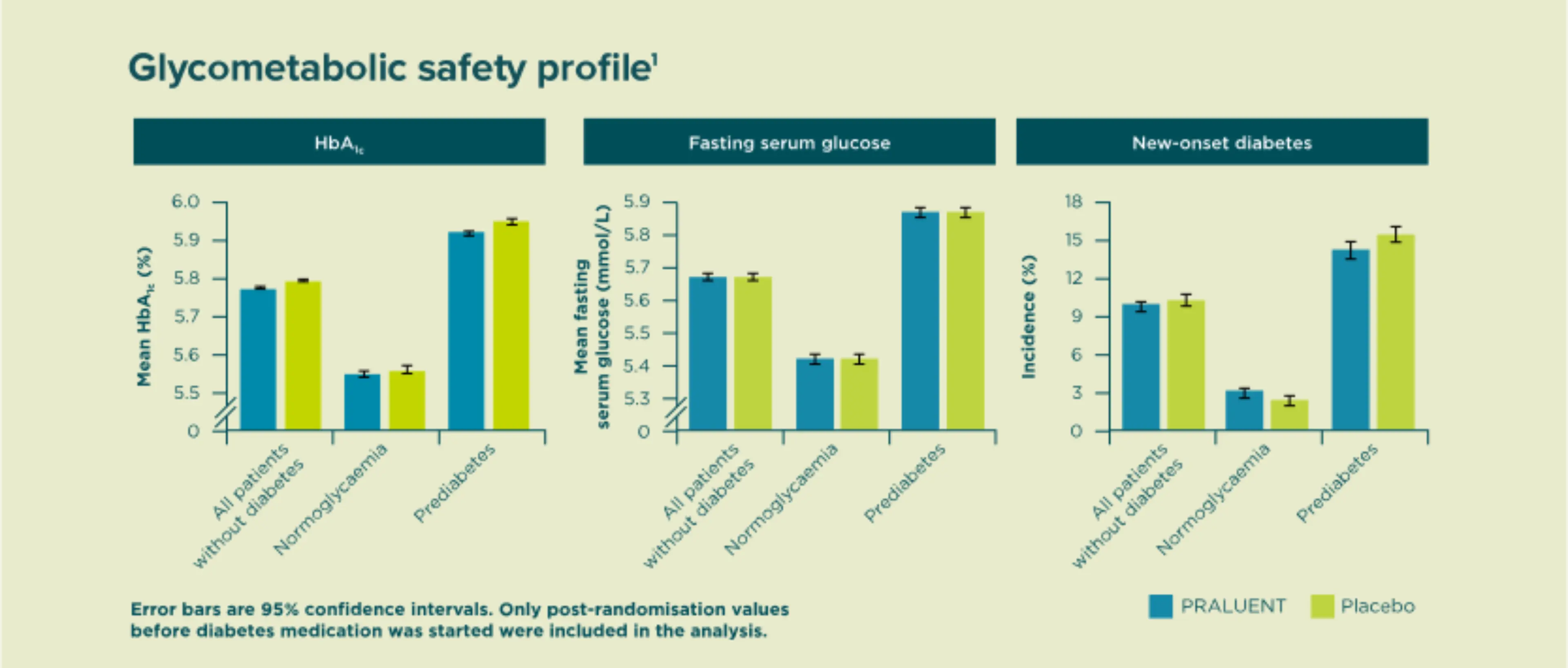
PRALUENT® did not increase the risk of new-onset diabetes for patients with prediabetes or normoglycemia at baseline1.
Praluent®
Find more information on Indication, Administration and Mechanism of Action and watch videos about Praluent®.
.png)
References
MACE=major adverse cardiac events (primary composite endpoint of CHD death, nonfatal myocardial infarction, fatal and nonfatal ischaemic stroke, or unstable angina requiring hospitalization; CV=cardiovascular; RRR=relative risk reduction; ARR=absolute risk reduction; CI=confidence interval; HR=hazard ratio; MI=myocardial infarction; LDL-C=low-density lipoprotein cholesterol.
1. Ray KK, et al. Lancet Diabetes Endocrinol 2019; 7:618-628
2. Schwartz G, et al. N Engl J Med 2018;379(22):2097
MAT-XU-2204592 (v3.0) Date of Preparation: October 2023