- Article
- Source: Campus Sanofi
- 23 Oct 2023
ODYSSEY OUTCOMES Trial Design Praluent® (alirocumab)

Odyssey Outcomes: a trial design with ~19,000 patients1
Trial overview
The ODYSSEY OUTCOMES trial involved 18,924 post-ACS patients, ~90% of whom were taking high-intensity statins:1†
RANDOMIZATION AT 1,315 SITES ACROSS 57 COUNTRIES1
2–5 YEARS DOUBLE-BLIND TREATMENT PERIOD1‡
2.8 YEARS MEDIAN FOLLOW-UP
Trial design
Designed to evaluate the long-term efficacy and safety of Praluent® in post-ACS patients3
PATIENTS WITH HIGH CV RISK: 100% WERE HOSPITALIZED WITH AN MI OR UNSTABLE ANGINA, 1-12 MONTHS PRIOR TO ENROLLMENT. 2.6 MONTHS WAS THE MEDIAN TIME POST INDEX EVENT TO RANDOMISATION1
ON TOP OF OPTIMAL STANDARD OF CARE: ~90% WERE ON HIGH-INTENSITY STATINS1
2.8 YEARS MEDIAN FOLLOW-UP; 44% WERE ELIGIBLE TO BE FOLLOWED FOR 3–5 YEARS;2 ~34% OF PATIENTS RECEIVED TREATMENT FOR AT LEAST 3 YEARS1
ODYSSEY OUTCOMES was a randomized, double-blind, placebo-controlled cardiovascular outcomes trial. Patients with a recent MI or unstable angina, and on high-intensity statin (40 or 80 mg atorvastatin or 20 or 40 mg rosuvastatin, or maximally tolerated dose of one of these agents) +/- other lipid-lowering therapy but not at predefined target LDL-C were enrolled.1
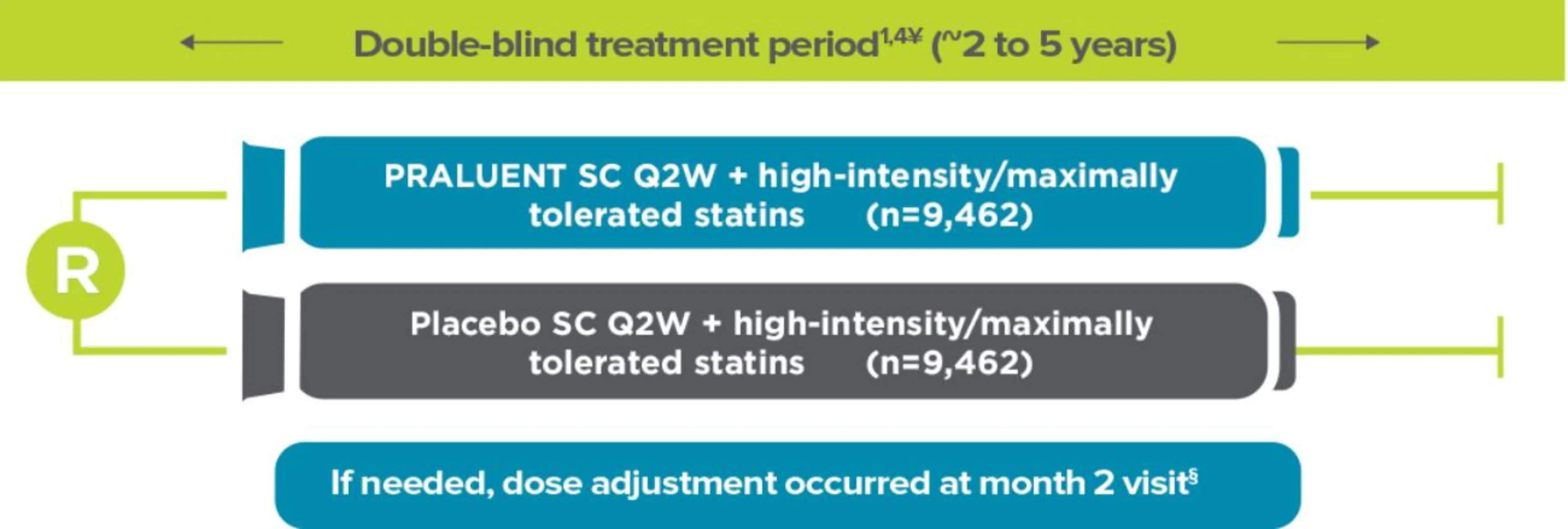
Adapted from Schwartz et al. 2018 and Schwartz et al.1,4
¥Background therapy: 96% aspirin, 88% P2Y12 inhibitor, 85% beta blocker, 78% ACE/ARBs.5§Dose was up-titrated from 75 mg Q2W to 150 mg Q2W at month 2 if LDL-C was ≥50 mg/dL at month 1. Dose was down-titrated from 150 mg Q2W to 75 mg Q2W if LDL-C was <25 mg/dL on 2 consecutive measures. Patients on the 75 mg Q2W dose were blindly switched to placebo if LDL-C was <15 mg/dL on 2 consecutive measures.
Trial endpoints
Primary endpoint (time to first occurrence of)3
-
MACE defined as:
-
CHD death, or
-
Nonfatal MI, or
-
Fatal and nonfatal ischemic stroke, or
-
Unstable angina requiring hospitalization
-
Major secondary endpoints (in order of hierarchical testing)3¶
-
CHD event (including CHD death, nonfatal MI, UA requiring hospitalization, and an ischemia-driven coronary revascularization procedure)
-
Major CHD event (including death from CHD and nonfatal MI)
-
CV event (including nonfatal MI and nonfatal ischemic stroke)
-
The composite of all-cause mortality, nonfatal MI, and nonfatal ischemic stroke
-
CHD death
-
CV death
-
All-cause death
Other secondary endpoints1
-
Components of the primary endpoint considered individually:
-
Time from randomization to first occurrence of any nonfatal MI
-
Time from randomization to first occurrence of fatal or any nonfatal ischemic stroke
-
Time from randomization to first occurrence of any unstable angina requiring hospitalization
-
-
Time from randomization to first occurrence of any ischemia-driven coronary revascularization procedure
-
Time from randomization to first occurrence of any congestive heart failure requiring hospitalization
†High intensity statins=atorvastatin 40 or 80 mg, rosuvastatin 20 or 40 mg daily, or the maximum tolerated dose of one of these agents.1
‡Patients from China were not followed for 2 years because a lengthy regulatory approval process delayed their random assignment to a trial group until after completion of the randomization process for the rest of the cohort.
¶ The hierarchy was revised during the course of the trial and was prespecified in the final statistical analysis plan. In the initial statistical analysis plan for the study, the prespecified testing hierarchy for secondary endpoints placed all-cause mortality directly after the composite endpoints of any coronary heart disease event, major coronary heart disease event, any cardiovascular event, and all-cause death, nonfatal myocardial infarction, or nonfatal ischemic stroke; cause-specific death did not appear in the hierarchy. In a subsequent amendment to the analysis plan that was implemented prior to unblinding of the study database, coronary heart disease death and cardiovascular death were placed ahead of all-cause death in the testing hierarchy, as reflected in the list of main secondary efficacy endpoints above.3
ACE=angiotensin converting enzyme; ACS=acute coronary syndrome; ARB=angiotensin receptor blocker; CHD=coronary heart disease; CV=cardiovascular; LDL-C=low-density lipoprotein cholesterol; MACE=major adverse cardiovascular events; MI=myocardial infarction; P2Y12 inhibitor=platelet signalling inhibitor; PCSK9i=proprotein convertase subtilisin/kexin type 9 inhibitor; Q2W=every two weeks; Q4W=every four weeks; R=randomization; SC=subcutaneous; SmPC=summary of product characteristics; UA=unstable angina.
Praluent®
Find more information on Indication, Administration and Mechanism of Action and watch videos about Praluent®.
.png)
References
- Schwartz G, et al. N Engl J Med 2018;379:2097–2107.
- Schwartz G, et al. Circulation 2019;140(2):103-112.
- Praluent Summary of Product Characteristics. Available at https://www.medicines.org.uk/emc/product/8093/smpc. Accessed October 2023.
- Schwartz G, et al. Am Heart J 2014;168(5):682–689.
- Schwartz G, et al. N Engl J Med 2018;379:2097–2107. Supplementary Appendix.
MAT-XU-2204592 (v3.0) Date of Preparation: October 2023