- Article
- Source: Campus Sanofi
- 23 Oct 2023
FH I & FH II Studies Praluent® (alirocumab)

ODYSSEY FH I and FH II: 78 week results with alirocumab treatment in 735 patients with heterozygous familial hypercholesterolaemia.
Kastelein JJP, et al. Eur Heart J 2015; 36:2996–3003.
All of the information and data within this section (unless noted) is a summary of this publication.
Study objectives
The ODYSSEY FHI and FH II studies evaluated the long-term efficacy and safety of Praluent® 75/150 mg in heterozygous familial hypercholesterolaemia‡ (HeFH) patients with inadequately controlled low-density lipoprotein cholesterol (LDL-C) despite maximally tolerated dose (MTD) statins** ± other lipid-lowering therapies (LLTs).
Study design
ODYSSEY FH I and FH II studies had similar study designs with FH I performed at 89 sites across North America, Europe and South Africa whilst FH II was performed across 26 sites in Europe.
Both FH I and FH II were 78 week, multicentre, double-blind, placebo-controlled, parallel group, phase III studies involving 735 adult HeFH‡ patients (FH I, n=486; FH II, n=249) who were receiving MTD statins** ± other LLTs with:
- LDL-C ≥1.8 mmol/L in patients with prior cardiovascular events†
or
- LDL-C ≥2.6 mmol/L in patients without a history of CV events
Patients were randomised to subcutaneous Praluent® 75 mg every 2 weeks (Q2W) (FH I, n=323; FH II, n=167) or placebo Q2W (FH I, n=163; FH II, n=82) (Figure 1). Praluent® dose was increased to 150 mg Q2W at Week 12 if Week 8 LDL-C was ≥1.8 mmol/L.
The primary efficacy endpoint was the percentage change in LDL-C from baseline to Week 24. Secondary endpoints included:
- Proportion patients achieving the pre-specified LDL-C level target of <1.8 mmol/L at Week 24
- Percentage change in LDL-C from baseline to Week 78
- Safety - including incidence of treatment-emergent adverse events (TEAEs), treatment-emergent serious AEs (SAEs), injection site reactions and adjudicated CV events.
Figure 1: ODYSSEY FHI and FH II: Study design - Adapted from Kastelein JJP, et al. Eur Heart J 2015; 36:2996–30031
.jpg)
Baseline characteristics
Baseline characteristics were well balanced between the treatment groups (Table 1). In FH I and FH II, 85.8% and 91.6% of Praluent patients received study treatment for ≥76 weeks versus 87.7% and 90.1% of placebo.
|
|
Praluent |
Placebo |
Praluent | Placebo (n=82) |
|
FH I |
FH II | |||
|
Demographics | ||||
|
Age in years, mean ±SD |
52.1 ± 12.9 |
51.7 ± 12.3 |
53.2 ± 12.9 |
53.2 ± 12.5 |
|
Male, % |
55.7 |
57.7 |
51.5 |
54.9 |
|
CV history/risk factors | ||||
|
CHD history*, % |
45.5 |
47.9 |
34.7 |
37.8 |
|
CHD risk equivalent†, % |
16.7 |
15.3 |
9.0 |
4.9 |
|
Type 2 diabetes, % |
9.9 |
15.3 |
4.2 |
3.7 |
|
Lipid medication | ||||
|
Any statin, % |
100 |
100 |
100 |
100 |
|
High dose statin§, % |
82.7 |
85.3 |
86.8 |
91.5 |
|
Ezetimibe, % |
56.0 |
59.5 |
67.1 |
64.6 |
|
Lipid parameters | ||||
|
LDL-C, (calculated) mmol/L ± SD |
3.7 ± 0.1 |
3.7 ± 0.1 |
3.5 ± 0.1 |
3.5 ± 0.1 |
HD = coronary heart disease; CV = cardiovascular; LLT = lipid-lowering therapy, SD= standard deviation.
*CHD history defined as acute myocardial infarction, silent myocardial infarction, unstable angina, or coronary revascularization.
†CHD risk equivalent; Ischaemic stroke, peripheral arterial disease, moderate chronic kidney disease (estimated glomerular filtration rate of ≥30 and ≤60 mL/min/1.73 m2), and diabetes with two or more risk factors.
§High-dose statin therapy defined as daily doses of 40–80 mg atorvastatin, 20–40 mg rosuvastatin or 80 mg simvastatin.
Key efficacy results
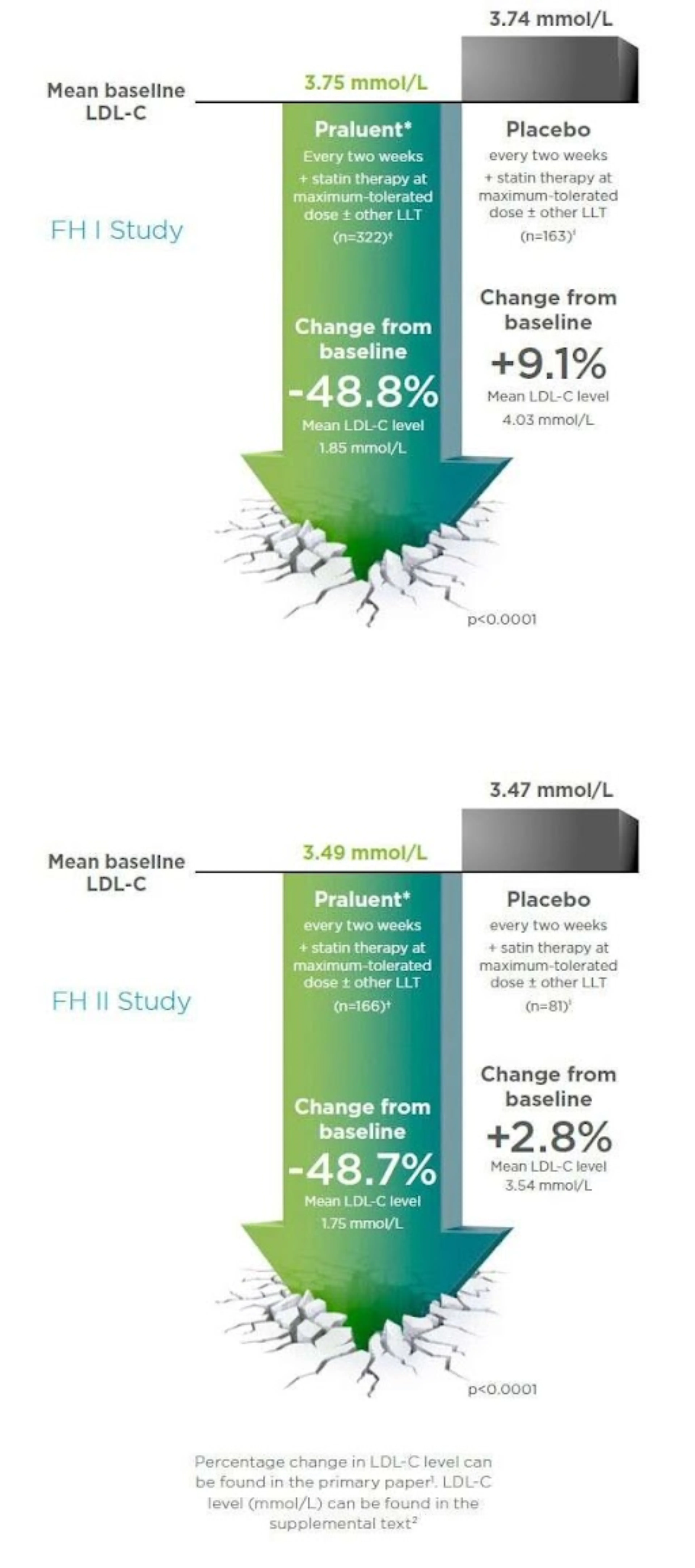
In adults with HeFH, Praluent75/150 mg 2QW led to significantly greater reductions in LDL-C compared to placebo (Figure 2), allowed a significantly greater proportion of patients to achieve a pre-specified LDL-C target compared to placebo (Figure 3) and LDL-C reductions were consistent and sustained over 78 weeks (Figure 4):
- At Week 24, mean percentage change in LDL-C was -48.8% (1.9mmol/L) (FH I) and -48.7% (1.74mmol/L) (FH II) for Praluent versus 9.1% (0.29mmol/L) (FH I) and 2.8% (0.07mmol/L) (FH II) for placebo (p<0.0001 for both studies) (Figure 2)
- At Week 24, regardless of prior CV status, 59.8% (FH I) and 68.2% (FH II) of Praluent patients achieved pre-specified LDL-C target of <1.8 mmol/L versus 0.8% (FH I) and 1.2% (FH II) of placebo patients (p<0.0001 for both studies) (Figure 3)
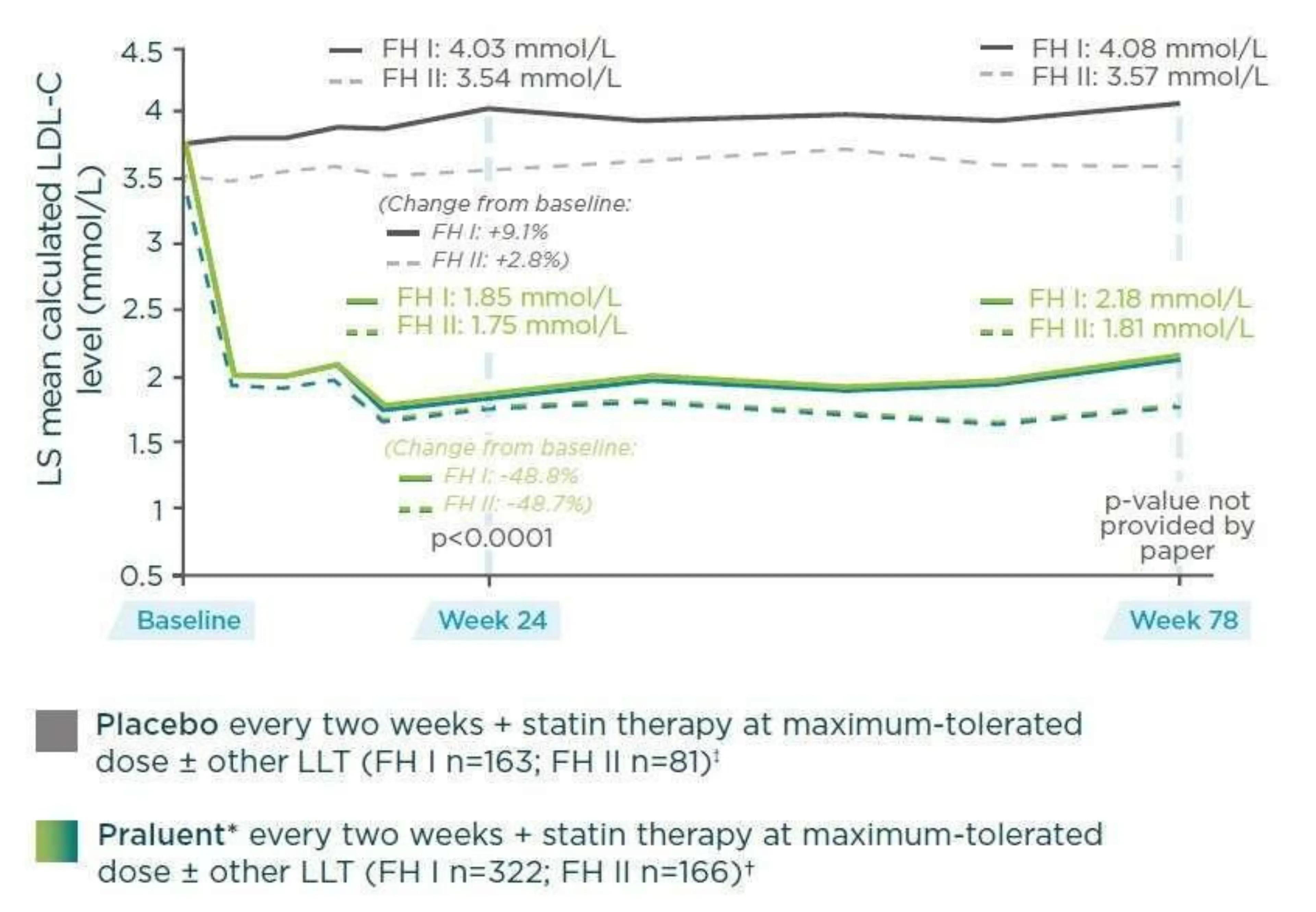
- At Week 78, the mean reduction in LDL-C level with Praluent compared with placebo was -51.8% (1.9mmol/L) (FH I) and -52.1% (1.76 mmol/L) (FH II) (Figure 4).
Amongst Praluent patients who received double-blind treatment for at least 12 weeks, 56.6% (FH I) and 61.4% (FH II) had LDL-C levels <1.8 mmol/L at Week 8 and therefore remained on Praluent 75 mg Q2W for the duration of the study. LDL-C responses were durable and were maintained (up to 78 weeks) in Praluent treated patients.
Figure 2: ODYSSEY FH I and FH II: Mean reduction in LDL-C from baseline to Week 52 - Adapted from Kastelein JJP, et al. Eur Heart J 2015; 36:2996–30031
Placebo + MTD statin therapy ± other LLT
Praluent + MTD statin therapy ± other LLT
*Praluent dose was 75 mg administered SC every two weeks, but if LDL-C level was ≥1.8 mmol/L at Week 8, a pre-specified dose up-titration to 150 mg every two weeks was performed at Week 12
†This number refers to the number of patients receiving Praluent (ITT analysis)1
‡This number refers to the number of patients receiving placebo (ITT analysis)1
Figure 3: ODYSSEY FH I and FH II: Proportion of patients achieving LDL-C target at Week 24 - (Adapted from Kastelein JJP, et al. Eur Heart J 2015; 36:2996–30031)
.jpg)
Placebo + MTD statin therapy ± other LLT
Praluent + MTD statin therapy ± other LLT
*Praluent dose was 75 mg administered SC every two weeks, but if LDL-C level was ≥1.8 mmol/L at Week 8, a pre-specified dose up-titration to 150 mg every two weeks was performed at Week 12
†This number refers to the number of patients receiving Praluent (ITT analysis)1
‡Intention to treat (ITT) analysis
Figure 4: ODYSSEY FH I and FH II: Mean reduction in LDL-C from baseline to Week 781,2 - (Adapted from Kastelein JJP, et al. Eur Heart J 2015;36:2996-3003)
Placebo + MTD statin therapy ± other LLT
Praluent + MTD statin therapy ± other LLT
‡Intention to treat (ITT) analysis
*Praluent dose was 75 mg administered SC every two weeks, but if LDL-C level was ≥ 1.8 mmol/L at Week 8, a pre-specified dose up-titration to 150 mg every two weeks was performed at Week 121
†Statin therapy at the maximum-tolerated dose (40-80 mg atorvastatin, 20-40 mg rosuvastatin daily or 80 mg simvastatin [if on this dose for >1 year]) or a lower dose, provided a reason was documented
Key safety results
Frequency of TEAEs and SAEs were similar in Praluent and placebo patients (Table 2). Although a higher proportion of Praluent than placebo patients experienced injection site reactions, these differences were non-significant (FH I, p=0.77; FH II, p=0.38); most reactions were mild and none lead to discontinuation (Table 2).
Table 2: ODYSSEY FH I and FH II: Treatment-emergent adverse events (safety population) - Adapted from Kastelein JJP, et al. Eur Heart J 2015; 36:2996–30031
|
|
Praluent |
Placebo |
Praluent | Placebo (n=81) |
|
FH I |
|
FH II |
| |
|
TEAE, n (%) |
263 (81.7) |
129 (79.1) |
125 (74.9) |
66 (81.5) |
|
Treatment-emergent SAEs, n (%) |
44 (13.7) |
22 (13.5) |
15 (9.0) |
8 (9.9) |
|
TEAE leading to discontinuation, n (%) |
11 (3.4) |
10 (6.1) |
6 (3.6) |
1 (1.2) |
|
Injection site reactions, n (%) |
40 (12.4) |
18 (11.0) |
19 (11.4) |
6 (7.4) |
|
TEAEs leading to death, n (%) |
6 (1.9) |
0 |
0 |
0 |
|
Safety events of interest | ||||
|
Positively adjudicated CV adverse events, n (%) |
8 (2.5) |
3 (1.8) |
2 (1.2) |
1 (1.2) |
|
General allergic TEAEs, n (%) |
28 (8.7) |
16 (9.8) |
19 (11.4) |
5 (6.2) |
|
Neurological TEAEs, n (%) |
12 (3.7) |
7 (4.3) |
7 (4.2) |
2 (2.5) |
|
Neurocognitive disorders, n (%) |
2 (0.6) |
2 (1.2) |
0 |
1 (1.2) |
|
Development/ |
6 (1.9) |
4 (2.5) |
4 (2.4) |
2 (2.5) |
|
Ophthalmologic disorders, n (%) |
3 (0.9) |
4 (2.5) |
3 (1.8) |
1 (1.2) |
|
Alanine aminotransferase, >3 x ULN (%) |
5/322 (1.6) |
2/163 (1.2) |
6/166 (3.6) |
1/81 (1.2) |
|
Creatine kinase, >3 x ULN (%) |
13/318 (4.1) |
10/163 (6.1) |
8/165 (4.8) |
6/80 (7.5) |
Please refer to publication for full safety profile information
CV = cardiovascular; SAEs, serious adverse events; TEAE = treatment-emergent adverse events
Conclusions
- Praluent 75/150 mg every 2 weeks significantly lowered calculated LDL-C from baseline to Week 24 in patients with HeFH on top of the maximum-tolerated dose of statin compared to placebo (-48.8% (1.9mmol/L) (FH I) and -48.7% (1.74mmol/L) (FH II) for Praluent versus 9.1% (0.29mmol/L) (FH I) and 2.8% (0.07mmol/L) (FH II) p <0.0001 for both studies)
- At Week 24, 59.8% of patients in the FHI study and 68.2% in the FHII study achieved the pre-specified target LDL-C level of <1.8 mmol/L compared to 0.8% (FHI) and 1.2% (FHII) patients on placebo (p<0.0001 for both studies)
- At week 8, over 50% of patients achieved pre-specified LDL-C target of <1.8 mmol/L and were maintained on the 75mg dose
- The incidences of TEAEs and treatment-emergent SAEs were similar between treatment groups over 78 weeks of treatment
‡HeFH diagnosed by either genotyping or clinical criteria (Simon Broome or World Health Organization/Dutch Lipid clinic network score of >8)
**Maximum-tolerated statin dose defined as 40–80 mg atorvastatin, 20–40 mg rosuvastatin or 80 mg simvastatin, or lower dose if documented reason provided e.g., intolerance or local practice
†Established CHD defined as acute myocardial infarction, silent myocardial infarction, unstable angina, coronary revascularisation, or clinically significant CHD diagnosed by invasive or non-invasive testing. CHD risk equivalents defined as ischaemic stroke, peripheral artery disease, moderate chronic kidney disease, or diabetes (only if ≥2 risk factors present)
§High-dose statin therapy defined as 40–80 mg atorvastatin, 20–40 mg rosuvastatin or 80 mg simvastatin
Praluent®
Find more information on Indication, Administration and Mechanism of Action and watch videos about Praluent®.
.png)
References
- Kastelein JJP, et al. ODYSSEY FH I and FH II: 78 week results with alirocumab treatment in 735 patients with heterozygous familial hypercholesterolaemia. Eur Heart J 2015; 36:2996–3003.
- Kastelein JJP, et al. ODYSSEY FH I and FH II: 78 week results with alirocumab treatment in 735 patients with heterozygous familial hypercholesterolaemia. Eur Heart J 2015; 36:2996–3003. Supplementary material.
MAT-XU-2204593 (v3.0) Date of Preparation: October 2023